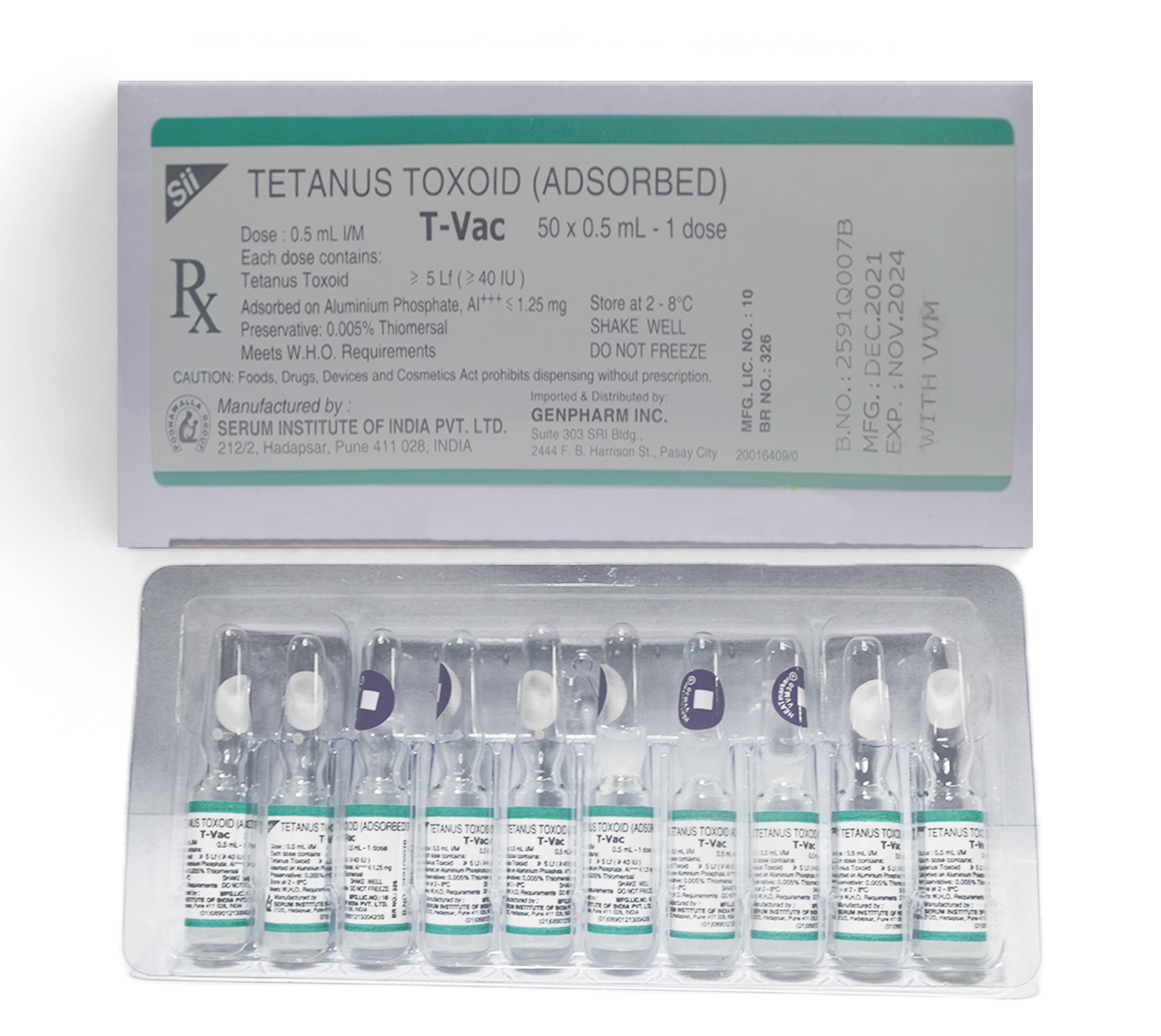
TETANUS TOXOID (ADSORBED) T-Vac
FORMULATION:
Each dose contains:
Tetanus Toxoid ≥ 5 Lf (≥ 40 IU)
Adsorbed on Aluminum Phosphate, Al+++ ⋜ 1.25 mg
Preservative : 0.005% Thiomersal
DOSAGE
The full basic course of immunization against tetanus consists of two primary doses of 0.5 mL at least four weeks apart, followed by the third dose 6-12 months later. To maintain a high level of immunity further 0.5 mL booster doses are recommended at every feasible interval (for adults usually 5 to 10 years).
STORAGE:
The vaccine should be stored in a dry, dark place at a temperature between 2-8∘C. Transportation should also be at 2-8∘C. DO NOT FREEZE.
Each dose contains:
Tetanus Toxoid ≥ 5 Lf (≥ 40 IU)
Adsorbed on Aluminum Phosphate, Al+++ ⋜ 1.25 mg
Preservative : 0.005% Thiomersal
DOSAGE
The full basic course of immunization against tetanus consists of two primary doses of 0.5 mL at least four weeks apart, followed by the third dose 6-12 months later. To maintain a high level of immunity further 0.5 mL booster doses are recommended at every feasible interval (for adults usually 5 to 10 years).
STORAGE:
The vaccine should be stored in a dry, dark place at a temperature between 2-8∘C. Transportation should also be at 2-8∘C. DO NOT FREEZE.
INDICATIONS:
The Vaccine is used for the prevention of tetanus in infants, children and adults, especially those lialble to be exposed to tetanus infection and persons engaged in outdoor activities e.g. gardeners, farm workers and athletes.
Tetanus toxoid vaccine is also used in the prevention of neonatal tetanus by immunizing women of childbearing age, and also in the prevention of tetanus following injury. The vaccine can be safely and effectively given simultaneously with BGC, Measles, Polio vaccines (IPV and OPV), Hepatitis B, Yellow fever vaccine, Haemophilus influenzae type b, Varicella vaccine and Vitamin A supplementation.
The Vaccine is used for the prevention of tetanus in infants, children and adults, especially those lialble to be exposed to tetanus infection and persons engaged in outdoor activities e.g. gardeners, farm workers and athletes.
Tetanus toxoid vaccine is also used in the prevention of neonatal tetanus by immunizing women of childbearing age, and also in the prevention of tetanus following injury. The vaccine can be safely and effectively given simultaneously with BGC, Measles, Polio vaccines (IPV and OPV), Hepatitis B, Yellow fever vaccine, Haemophilus influenzae type b, Varicella vaccine and Vitamin A supplementation.
ADRENALINE INJECTION (1:1000) MUST BE IMMEDIATELY AVAILABLE SHOULD AN ACUTE ANAPHYLACTIC REACTION OCCUR DUE TO ANY COMPONENT OF THE VACCINE. For treatment of severe anaphylaxis the initial dose of adrenaline is 0.1-0.5 mg 90.1-0.5 mL of 1:1000 injection) given s/c or i/m. Single dose should not exceed 1 mg (1 mL). For infants and children the recommended dose of adrenaline is 0.01 mg/kg (0.01 mL/kg of 1:1000 injection). Single paediatric dose should not exceed 0.5 mg (0.5 mL). The mainstay in the treatment of severe anaphylaxis is the prompt use of adrenaline, which can be lifesaving. It should be used at the first suspicion of anaphylaxis.
As with the use of all vaccines the vaccinees should remain under observation for not less than 30 minutes for possibility of occurrence of immediate or early allergic reactions. Hydrocortisone and antihistaminics should also be available in addition to supportive measures such as oxygen inhalation.
There is an increased incidence of local and systemic reactions to booster doses of tetanus toxoid when given to previously immunized persons.
Special care should be taken to ensure that the injection does not enter a blood vessel.
IT IS EXTREMELY IMPORTANT WHEN THE PARENT, GUARDIAN, OR ADULT PATIENT RETURNS FOR THE NEXT DOSE IN THE SERIES, THE PARENT, GUARDIAN, OR ADULT PATIENT SHOULD BE QUESTIONED CONCERNING OCCURANCE OF ANY SYMPTOMS AND/ OR SIGNS OF AN ADVERSE REACTION AFTER THE PREVIOUS DOSE.
As with the use of all vaccines the vaccinees should remain under observation for not less than 30 minutes for possibility of occurrence of immediate or early allergic reactions. Hydrocortisone and antihistaminics should also be available in addition to supportive measures such as oxygen inhalation.
There is an increased incidence of local and systemic reactions to booster doses of tetanus toxoid when given to previously immunized persons.
Special care should be taken to ensure that the injection does not enter a blood vessel.
IT IS EXTREMELY IMPORTANT WHEN THE PARENT, GUARDIAN, OR ADULT PATIENT RETURNS FOR THE NEXT DOSE IN THE SERIES, THE PARENT, GUARDIAN, OR ADULT PATIENT SHOULD BE QUESTIONED CONCERNING OCCURANCE OF ANY SYMPTOMS AND/ OR SIGNS OF AN ADVERSE REACTION AFTER THE PREVIOUS DOSE.
SERUM INSTITUTE OF INDIA PVT. LTD.
212/2, Hadapsar, Pune 411028, India
212/2, Hadapsar, Pune 411028, India




